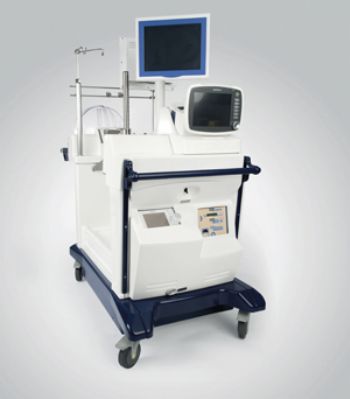
The US Food and Drug Administration (FDA) has approved the XVIVO Perfusion System (XPS) — a device that preserves donated lungs that do not initially meet the criteria for use in transplants and require further evaluation.
Christy Foreman, director of the Office of Device Evaluation at the FDA’s Centre for Devices and Radiological Health, said: “With this approval, there may now be more lungs available for transplant, which could allow more people with lung disease who have exhausted all other treatment options to receive a transplant.”
With additional time to determine if a donated lung meets the criteria for lung transplantation, the XPS can be used to ventilate the donor lungs and warm them to near normal body temperature.
Donor lungs can stay in the machine for up to 4hr; during this time, the transplant team can examine them and evaluate their function. If they meet certain functionality criteria, they can then be transplanted into a recipient.