Croom Precision Medical (CPM –
www.croomprecision.com) has chosen Renishaw (
www.renishaw.com) as its additive-manufacturing (AM) and metrology partner; it uses the latter’s technology throughout the production and validation of its ISO13485-certified medical devices.
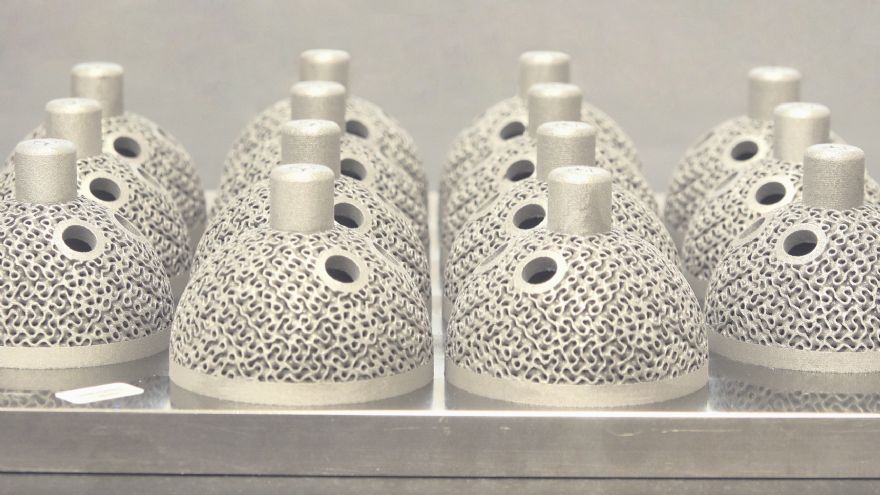
CPM has found that Renishaw’s AM technology allows it to incorporate complex features into implant designs at a commercially viable cost, while also maintaining traceability throughout the manufacturing process, which is essential in the heavily regulated medical environment.
Patrick Byrnes, research and development manager at CPM, said: “We want to achieve consistency and reliability in the products that we make. CPM has a vision of becoming a centre of excellence for additive manufacturing in Ireland.”
The company has invested in a RenAM 500M additive-manufacturing system, which it uses to 3-D print medical devices in titanium; and because Renishaw’s AM technology can produce complex structures in one manufacturing operation, it has begun assessing the use of gyroid lattices on acetabular cups — a design feature that would not have been economically feasible before the company’s investment in AM.
Mr Byrnes says that Renishaw’s inline process monitoring gives CPM a competitive edge: “The Renishaw software provides us with all the necessary information to confirm the quality of our parts, while giving us traceability of the build process from start to finish.”