Germany-based
Micrometal GmbH, a pioneer in the development of the photo chemical etching (PCE) process, has once again been awarded with ISO 13485 accreditation, critical in the company’s mission to bring its next-generation precision metal fabrication technology to all relevant sectors of industry.
Micrometal’s years working in the medical device sector has demonstrated the imperative for flawless precision metal parts and components due to the critical nature of these devices. Even the slightest imperfections can lead to malfunctions, compromised patient safety, and regulatory non-compliance. Therefore, ensuring perfection is vital to maintain the reliability and efficacy of medical devices, supporting accurate diagnoses, treatments, and patient outcomes.
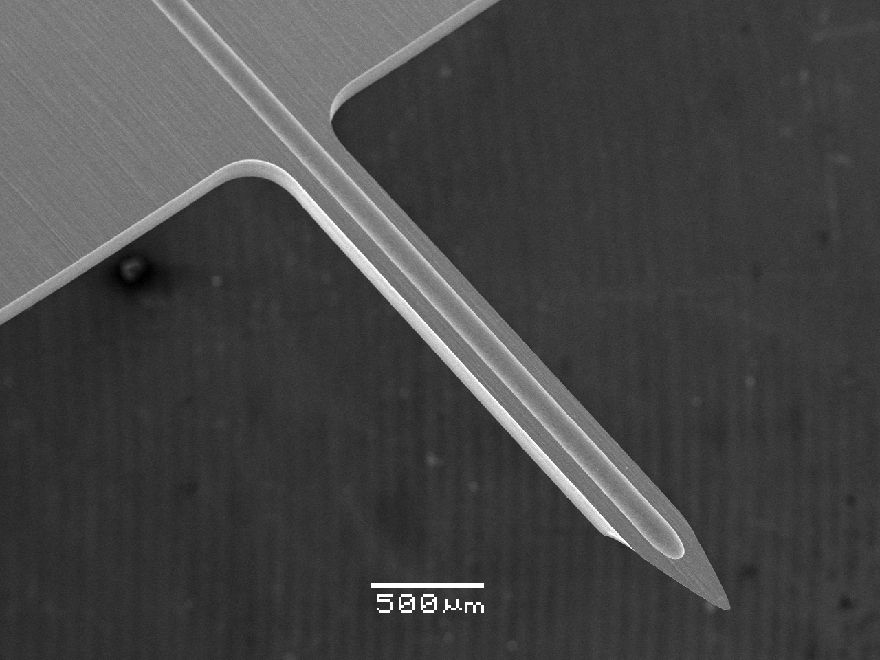
The company’s PCE process stands out as an ideal technology for achieving this level of precision. Its intricacy allows for the production of highly detailed and complex parts with minimal material stress, preserving the integrity of the metal and eliminating the risk of burrs or distortions. Additionally, the process offers exceptional repeatability and tight tolerances, guaranteeing consistent quality across large-scale production. This makes PCE exceptionally well suited for crafting intricate, burr-free, and dimensionally accurate parts that adhere to the stringent requirements of the medical device sector.
Jochen Kern, head of sales and marketing at Micrometal, said: “ISO 13485 accreditation is of paramount importance for a sub-contract precision metal parts manufacturer like Micrometal, as it signifies our compliance with rigorous quality management standards specific to the medical device industry. This accreditation demonstrates our commitment to producing components via our next-generation PCE process that adhere to the highest levels of quality, safety, and traceability, critical for medical devices where precision, reliability, and consistency are non-negotiable.”
Industry best practicesHe continued: “ISO 13485 not only enhances our credibility and marketability within the healthcare sector, but also ensures seamless collaboration with medical device companies by aligning their processes with industry best practices, ultimately contributing to the delivery of safe and effective medical products.”
The accreditation is a quality management standard specifically tailored for the medical device industry, and was developed to address the unique regulatory and safety demands of this sector. Medical devices directly impact human health and safety, necessitating a comprehensive framework that ensures consistent adherence to stringent quality controls. ISO 13485 was conceived to provide a standardised approach to quality management, encompassing design, development, production, and post-market activities, while considering the critical aspects of risk management, process validation, and traceability that are paramount in healthcare applications.
By establishing a clear and harmonised set of guidelines, ISO 13485 facilitates compliance with global regulatory requirements, streamlines communication across the supply chain, and instills confidence in the safety and reliability of medical devices.
Mr Kern continued: “From our perspective at Micrometal, ISO 13485 certification underscores a profound dedication to the medical device sector and signifies a superior level of service. By meeting the stringent requirements of this industry-specific quality management standard, we have demonstrated our unwavering commitment to delivering products and services of the highest quality, precision, and reliability.”
He concluded: “ISO 13485 not only ensures compliance with rigorous regulatory standards but also showcases a proactive approach to risk management, process optimisation, and continuous improvement. This commitment enhances trust among medical device manufacturers and end-users, affirming our capability to contribute to the development and production of safe and effective medical devices, thereby solidifying our position as a valued partner in the healthcare ecosystem.”