Fenton Precision Engineering — a tool-maker and injection moulder —was recently tasked by Cambridge-based RBR Active Ltd with developing a potentially life-saving medical device.
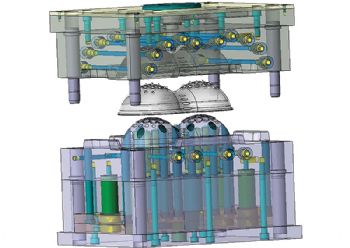
The company then designed and machined the complex steel mould tool — comprising some 30 components — to produce the device, which will reduce the possibility of the user suffering a deep-vein thrombosis (DVT).
Injection-moulded from polypropylene on a 120-tonne Yizumi press, the RBR Legflow device is said to be ‘effective anywhere’ during long periods of inactivity, such as air travel, working in an office, being in hospital or just prolonged sitting.
Carrying out three simple foot exercises with it leads to an 11-fold increase in blood flow to the lower limbs.
The mould tool for it was designed using VISI software (
www.visicadcam.com) and cut from P20 tool steel on a Dugard CNC machine programmed with WorkNC (
www.worknc.com) — both software solutions from Hexagon Manufacturing Intelligence’s Production Software business.
When RBR Active’s managing director, Paul Westerman, approached Fenton, he asked if it could produce a mould tool based on the over-moulded concept.
However, Richard Brown — Fenton’s technical director — said: “We suggested it would be better to mould it as a one-piece product.
“For this approach, VISI’s reverse-engineering capability was essential, allowing us to take the point data from our CMM, combine surfaces and solids, and seamlessly work between the two.”
The wall section at the top of the product’s two dome structures, which contains 38 nodules, has to be particularly thin to allow flexibility.
“However, there is obviously a limit to how thin we could make the wall. We got the optimum thickness using VISI Flow Lite, which simulates the filling phase of the injection-moulding process.
“Design engineer Martin Edwards defined all the initial moulding criteria and gating positions with it, allowing him to achieve a well-balanced filling of the cavities under optimum moulding conditions.
“Once he was happy with that, we had 3-D prints of the product made; and after a few minor design changes, Mr Westerman signed it off.
“We constructed the tool around this ‘print’ using VISI Mould, which has a built-in Meusburger catalogue for the ‘native’ parts.
“Because of the radius shape of this product, our CAD designers decided that the holes for the eight ejector pins should be produced using our wire eroder after the main surface had been machined on the Dugard VMC.
“Both sets of tool-paths were generated using WorkNC.”
Mr Brown describes the mould tool as being “straight open and close,” with two cavity plates, support plates, and back plates making up some 30 different components.
He added: “One of the design challenges was to ensure there were no ‘side actions or lifters’.”
Mould manufacture
The manufacture of the complete tool took around six weeks. Each plate for the cores and cavities took about 25hr to machine, followed by 20hr for the build.

“It was quite a complex format, with a lot of small solid-carbide cutters from a variety of suppliers used for machining.
“We also used shrink-fit tooling to ensure greater accuracy, balanced chip loads and better finishes, as well as increased speed and feed rates.
“Moreover, WorkNC’s ability to produce high-quality surface finishes kept subsequent surface polishing to a minimum.”
The full time-line for the overall project was around 11 months: June 2018, initial enquiry from RBR Active; October 2018, order for 3-D printing; December 2018, product reviewed and approved, and mould tool order placed; January 2019, initial mould tool design completed; March 2019 (following ‘final amends’), mould tool approved; May 2019, first production run of the injection-moulded products.
On Fenton’s recommendation, SteriTouch anti-microbial ‘master-batches’ were added to the moulding material.
These are suitable for all plastics and polymers (including PVC, PC/ABS and TPE); at low addition rates, they offer ‘excellent protection’ against bacteria, biofilm, fungi and mould for the lifetime of the final product.
Gwent-based SteriTouch says its anti-microbial master-batch requires no changes to the manufacturing process and can be added at the ‘dosing stage’, without affecting the appearance of the end product.
Mr Brown said that while the extensive range of functionality in both VISI and WorkNC combined to ensure that the project was completed successfully, VISI’s mould package and its built-in catalogues of mould tool suppliers (such as Meusburger and Hasco) were critical at the design stage, while WorkNC’s surface finishing and ability to program small cutters with precise tool-paths were critical at the manufacturing stage.
“WorkNC readily reads native VISI files, so it’s a seamless transition from CAD to CAM. Once we save the VISI work-file, we simply open it in WorkNC.”
As well as working with the medical industry, Fenton provides parts for the building sector, automotive (engines and interiors), health-care, cosmetics, white goods, packaging and retail.
The company’s tool-room and main moulding facility are in Northamptonshire, with an additional moulding factory in Leicestershire.
It has a total of 33 moulding machines across the two sites, and it has invested heavily in Yizumi injection presses, along with those from Negri Bossi, Arburg, Borche and Demag. Capacities range from 25 tonnes to 500 tonnes.